Medical Metrics, Inc. is proud to have worked with Optinose on their ReOpen clinical trials, which have recently received FDA approval! A heartfelt thank you to all our amazing collaborators, including our dedicated neuroradiologist readers, Key Opinion Leader consultants, and the incredible project personnel on both teams!
Press Release transcript below:
HOUSTON, TEXAS, UNITED STATES, May 13, 2024 /EINPresswire.com/ — Medical Metrics, Inc. (MMI) is proud to have partnered with Optinose® on their chronic rhinosinusitis (CRS) clinical program and congratulates the Optinose® team on receiving U.S. Food and Drug Administration (FDA) approval for the XHANCE® nasal spray after the ReOpen clinical trials. MMI also extends its thanks and appreciation to its key opinion leaders, independent radiologist reviewers, and scientific consultants who were instrumental in running a compliant and high-quality image analyses program in support of the ReOpen trials.
CRS affects up to 16% of the United States population, triggering both direct healthcare burden and indirect costs to quality of life. The total economic burden to the US has been estimated to be around $65 billion (Caulley et al, 2015). This substantial consumer base fuels ongoing innovation in rhinology, driving the search for interventions and treatments for long-term management of CRS.
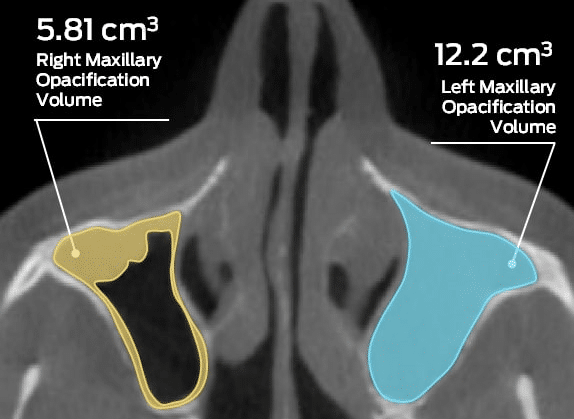
Visual, categorical CT-based scoring systems such as Lund-Mackay (LM) or its Zinreich Modification have been in use for several years for qualitative CRS staging. Yet, capturing change in treatment effects based on such grading systems may be suboptimal due to the broadness of each categorical grade level. The subjective nature of these evaluations may not necessarily correlate with a patient’s clinical symptoms (Garneau et al, 2015). Ultimately, the effectiveness of categorical techniques utilized to monitor long-term changes will influence imaging outcomes, underscoring the necessity for more objective measures. This need to objectively quantify and characterize CRS disease progression (e.g., changes in mucosal thickening, percent opacification of sinuses, etc.) has elicited a paradigm shift in treatment development and research into characterizing the efficacy of treatments.
In an effort to objectively measure CRS treatment effects, MMI developed a validated, semi-automated 3D analysis methodology to measure mucosal thickening volumes of the sinuses utilizing thin-slice CT scans. The advantages of volumetric analysis are many-fold. Using a volumetric approach permits greater objectivity, correlates better with clinical symptoms, and provides higher sensitivity to detect changes over time (Pallanch et al, 2013). Reacting to the recent FDA approval of the XHANCE® nasal spray (ReOpen) trials that used volumetric analysis as a co-primary endpoint, MMI Director of Client Services, Vijay Ramu, MS, MBA stated: “Volumetric analysis is quickly becoming the gold standard for characterization of CRS due to its ability to provide accurate, reliable, sensitive, and reproducible results. This is a game changer for the rhinologic community with its potential to detect differences that might otherwise be missed using conventional categorical grading systems.”
About Medical Metrics:
Medical Metrics, Inc. is an ISO 9001:2015-certified provider of independent imaging core lab services and a leading core lab for clinical trials and associated scientific consulting. MMI assists sponsors with designing and executing the imaging strategy of their global trials through our responsive trial management team, robust operating infrastructure, and world-class imaging expertise. More information about MMI’s Otolaryngology expertise and its Services can be found at www.medicalmetrics.com.
References:
Caulley, L., Thavorn, K., Rudmik, L., Cameron, C., & Kilty, S. J. (2015). Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: Results of the US Medical Expenditure Panel Survey. In Journal of Allergy and Clinical Immunology (Vol. 136, Issue 6, pp. 1517–1522). Elsevier BV. https://doi.org/10.1016/j.jaci.2015.08.037
Garneau, J., Ramirez, M., Armato, S. G., III, Sensakovic, W. F., Ford, M. K., Poon, C. S., Ginat, D. T., Starkey, A., Baroody, F. M., & Pinto, J. M. (2015). Computer‐assisted staging of chronic rhinosinusitis correlates with symptoms. In International Forum of Allergy & Rhinology (Vol. 5, Issue 7, pp. 637–642). Wiley. https://doi.org/10.1002/alr.21499
Pallanch, J. F., Yu, L., Delone, D., Robb, R., Holmes, D. R., III, Camp, J., Edwards, P., McCollough, C. H., Ponikau, J., Dearking, A. C., Lane, J., Primak, A., Shinkle, A., Hagan, J., Frigas, E., Ocel, J. J., Tombers, N., Siwani, R., Orme, N. M., … Kita, H. (2013). Three‐dimensional volumetric computed tomographic scoring as an objective outcome measure for chronic rhinosinusitis: clinical correlations and comparison to Lund‐Mackay scoring. In International Forum of Allergy & Rhinology (Vol. 3, Issue 12, pp. 963–972). Wiley. https://doi.org/10.1002/alr.21219
Latest Industry News
[Press Release] Driving Excellence in Cardiovascular Trials: Medical Metrics, Inc and Healthcare Inroads, LLC Deepen Collaboration