Since 2000, the FDA has explicitly described the importance of radiographic evaluation in spinal studies in primary and secondary endpoints.
Key Highlights
- For spine clinical trials, the FDA recommends a minimum of 2 years of radiographic imaging and follow-up data.
- Radiographic evaluation should be done at Baseline and at all Post-Operative time points.
- A “2+1” reviewer paradigm is standard for determination of fusion reads.
The role and importance of imaging is often understated in the clinical trial environment. With the complexities of site and subject logistics combined with the cost and time constraints, something as seemingly routine as an x-ray is often overlooked. However, the FDA begs to differ when it comes to Spinal devices. In 2000, the FDA issued a formal Guidance Document detailing the “Preparation of IDEs for Spinal Systems”, which continues to serve as a primary manual to successfully run trials to date. This Guidance Document asserts imaging as a key component of a successful Spine trial.
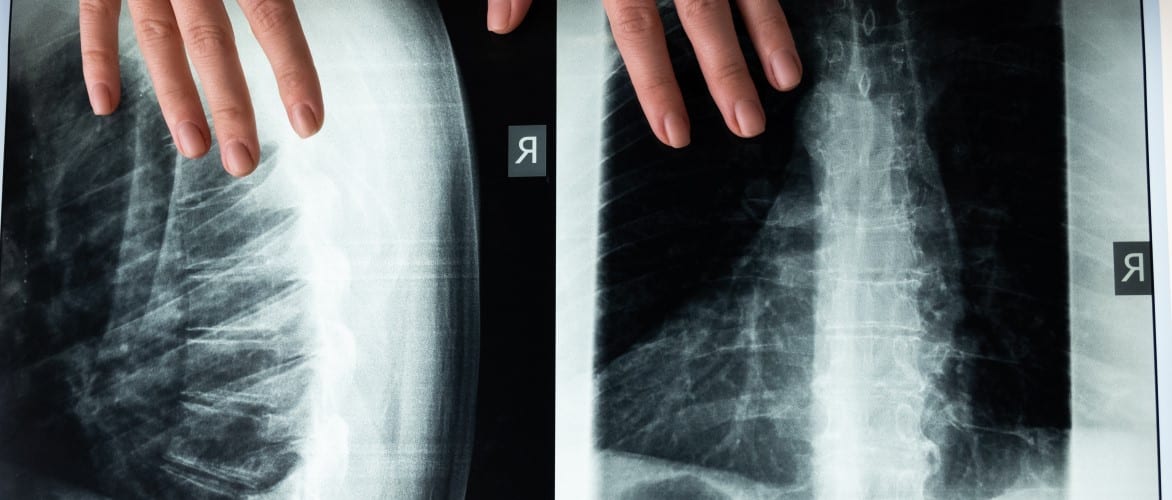
Imaging often serves as a major component of the subject screening process to enroll individuals into a given study. Depending on the treatment being investigated, radiographic findings can be a major gatekeeper. For example, in degenerative disc disease, confirmatory radiographic measurements of instability, osteophyte formation, decreased disc height, scarring/thickening, disc herniation, joint degeneration, etc. are expected by the FDA during subject enrollment.
Longitudinal radiographic data is crucial as well. The FDA prefers spinal studies with at least 2 years of follow-up, comparing radiographs taken Pre-Operatively, 6 weeks, 3 months, 6 months, 12 months, 24 months, and annually until the final subject’s 2-year follow-up is completed.
Depending on the type of spinal system, radiographic evidence of fusion or non-fusion is a crucial primary parameter to include. Measurement of disc or vertebral height is an important secondary parameter. Per FDA guidance, the radiographic determination of fusion “should be read by at least two radiologists”, with a third to adjudicate any necessary tie-breaks. Assessment of fusion should be based on findings such as evidence of bridging bone and limited translational and angular motion. Assessment of radiolucent lines is also necessary. Conversely, motion preservation devices require assessment of non-fusion, including lack of bridging bone and an appropriate range of motion for the spinal level.
MMI is a leading core lab for spine clinical trials, a reputation built up over two decades of recognizing the fundamental importance of imaging in spine studies. Our extensive history in the space provides clients the expertise necessary to fully address the imaging concerns of regulatory agencies, both in the US and around the world. MMI can work with a sponsor from day 1 to build a protocol that synergizes with a trial’s imaging needs and to implement imaging analysis and other core lab duties seamlessly with the rest of the study.
Read the full FDA Guidance Document here.
Medical Metrics, Inc. is an ISO 9001:2015-certified provider of independent imaging core lab services. We assist sponsors with designing, implementing, and executing the imaging strategy of their global trials through our responsive trial management team, robust operating infrastructure, and world-class imaging expertise. Learn more about our Spine expertise and our Services.