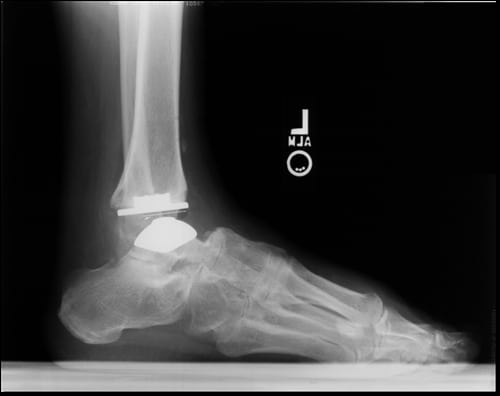
Faced with a rapidly aging population, the U.S. health care industry has dramatically increased its focus on treatments that are able to improve quality of life in later years. With millions of Americans currently suffering from arthritis and tens of thousands reaching the age considered to be most at risk every day, increased research and innovative treatments are sorely needed to address this condition.
A team of researchers from Emory University School of Medicine in Atlanta, GA, led by Dr. Jason T. Bariteau, recently announced their findings at the 2015 Annual Meeting of the American Academy of Orthopedic Surgeons. His team found that for patients over 70 years of age with end-stage ankle arthritis, total ankle arthroplasty (TAA) was found to improve gait parameters, granting elderly patients greater mobility and quality of life.
The study first looked at TAA outcomes for 21 older patients, then compared those against the results of 21 younger patients who underwent a TAA procedure between 1999 and 2010. The same surgeon performed every procedure included in the study.
According to OrthoSpineNews, TAA has become an increasingly popular procedure to help manage the effects of end-stage ankle arthritis. Dr. Bariteau’s findings are worthy of note as they establish that older patients, the fastest growing age group, can realize the same benefits as their younger counterparts. TAA also holds several possible advantages over alternative treatments, such as ankle arthrodesis, that the study identified. Some examples include a decrease in long-term adjacent midfoot and hindfoot arthritis and an improvement in gait metrics, according to Dr. Bariteau.
Staying abreast of the most recent findings related to arthritis treatment is valuable not only for providers, but for medical device manufacturers and developers as well. Knowing the types of procedures that hold the most benefit for patients can help ensure that future devices serve an immediate need in the market. Partnering with a CRO clinical research firm with a history in approving devices aimed at addressing arthritis can also increase chances of both FDA approval and widespread adoption by physicians.
Medical Metrics, Inc., recently provided our expert medical imaging services to the first clinical trial of a total ankle arthroplasty device to be approved in the U.S., the STAR ankle implant. To date, it remains the only pre-market approved three-piece mobile bearing non constrained, uncemented total ankle replacement available to post traumatic arthritis or rheumatoid arthritis sufferers. It has so far been implanted in over 15,200 patients worldwide.
The combination of our industry-leading medical imaging technologies and record of success with the approval of novel products that improve quality of life for arthritis sufferers makes Medical Metrics an ideal resource for trial sponsors. Our lengthy history of prior approvals allows us to offer the ideal imaging strategies and equipment to facilitate the timely approval of related devices.
Latest Industry News
[Press Release] Driving Excellence in Cardiovascular Trials: Medical Metrics, Inc and Healthcare Inroads, LLC Deepen Collaboration
Medical Metrics, Inc. Announces Involvement in ReOpen CRS Clinical Trials