MMI Co-Founder, John Hipp, PhD. to be Guest Editor for Special Issue of Bioengineering! Submit by May 30!
Learn MorePress Release!: Driving Excellence in Cardiovascular Trials: MMI and Healthcare Inroads Deepen Collaboration
Read HereThe MMI & Micron Advantage
Diverse Cancer Experience
We have a wide range of expertise for oncological central imaging review, supporting Phase I, II, III, and Post-Market studies, for solid tumors and hematological cancers.
Beyond the RECIST 1.1
Gold Standard
In addition to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 method and its modifications, we support unique assessments including 3D tumor volume and more.
Comprehensive PET Support
As a trailblazer for PET imaging standardization in East Asia, Micron's full-service PET support now extends worldwide in tandem with MMI.
Experience and Expertise
Modality Expertise
CT
MRI
PET
SPECT
Gamma Camera
Endoscope
Oncology expertise
Bladder
Brain
Breast
Colorectal
Esophageal
Kidney
Leukemia
Liver
Lung
Malignant Lymphoma
Melanoma
Mesothelioma
Neuroendocrine
Ovarian
Pancreatic
Prostate
Thyroid
This unparalleled partnership provides numerous unique and powerful capabilities. Global coverage ensures rapid turnaround time requirements crucial to oncology trial needs. Our PET imaging specialists have extensive experience with a wide range of radiotracers and radionuclides (18F, etc.). We are also able to provide site qualification and phantom testing services to confirm site imaging acquisition capabilities for all major modalities, including PET. Our emphasis on Current Good Practices (cGxP) ensures consistent compliance and quality processes.
WHAT WE OFFER
Response Evaluation Criteria in Solid Tumors (RECIST) 1.0/1.1
Modified RECIST (mRECIST) for hepatocellular carcinoma (HCC) & malignant pleural mesothelioma (MPM)
irRECIST and iRECIST
Immune-related Response Criteria (irRC)
PET Response Criteria in Solid Tumors (PERCIST)
Response Evaluation Criteria in Cancer of the Liver (RECICL)
Cheson guidelines (1999, 2007)
Lugano classification
Response Evaluation Criteria in Lymphoma (RECIL)
Response Assessment in Neuro-Oncology (RANO)
Prostate Trials Working Group guidelines (PCWG2 & PCWG3)
National Cancer Institute Working Group guidelines (NCIWG)
International PCNSL Collaborative Group guidelines (IPCG)
International Workshop on Waldenstrom’s Macroglobulinemia guidelines (IWWM)
Tsukasaki criteria
World Health Organization criteria (WHO)
3D Tumor Volume
Organ-Specific Response Rate (OSRR)
Tumor Growth Rate
Dosimetry analysis
Dynamic Contrast Enhanced MRI (DCE-MRI) analysis
PET tracer studies
Theranostic studies
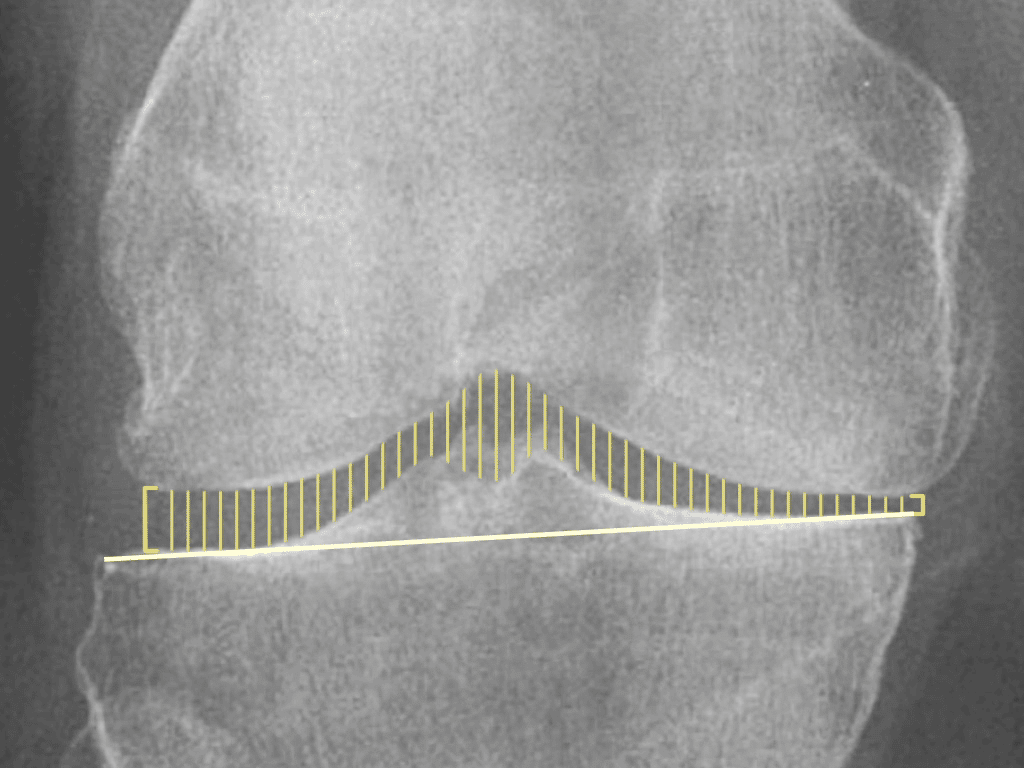
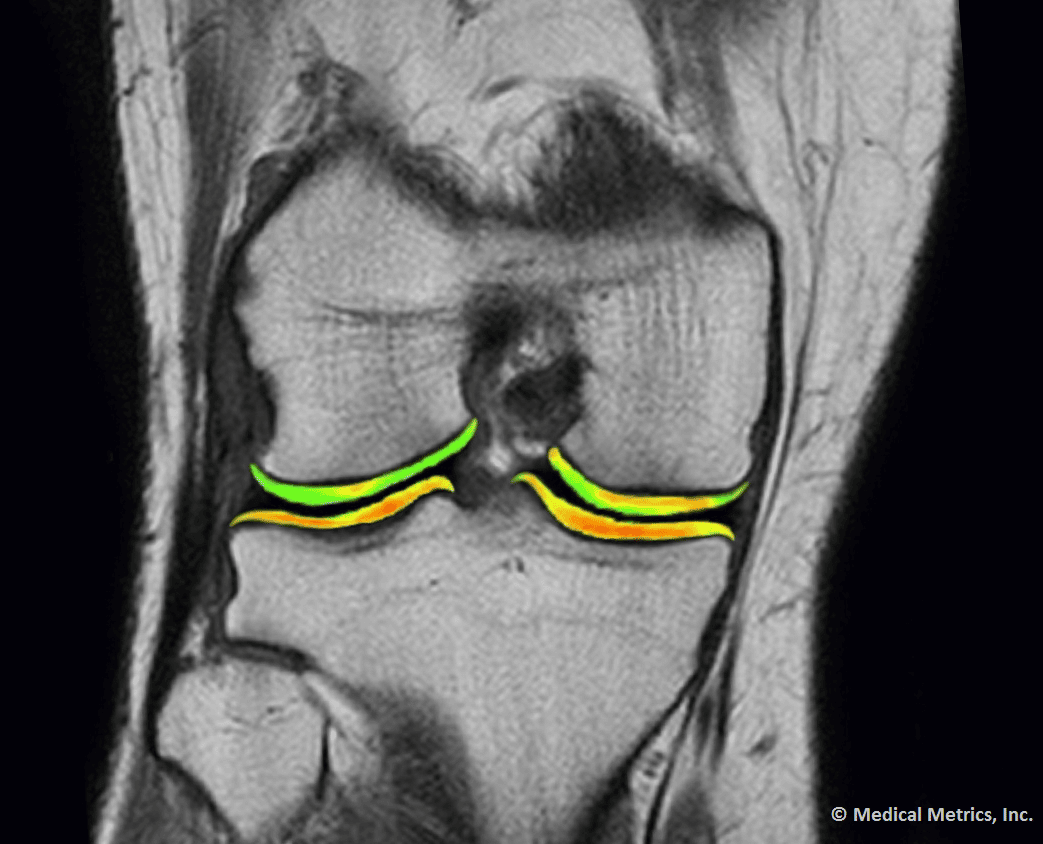
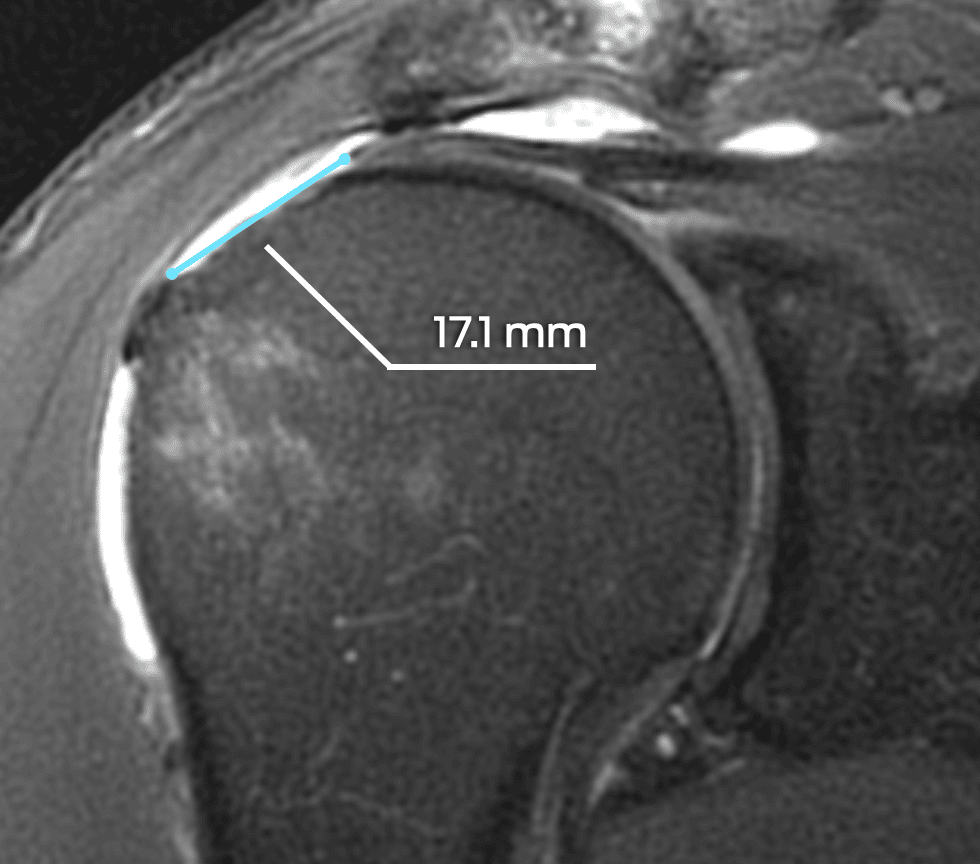
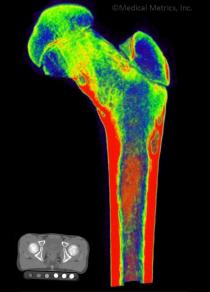
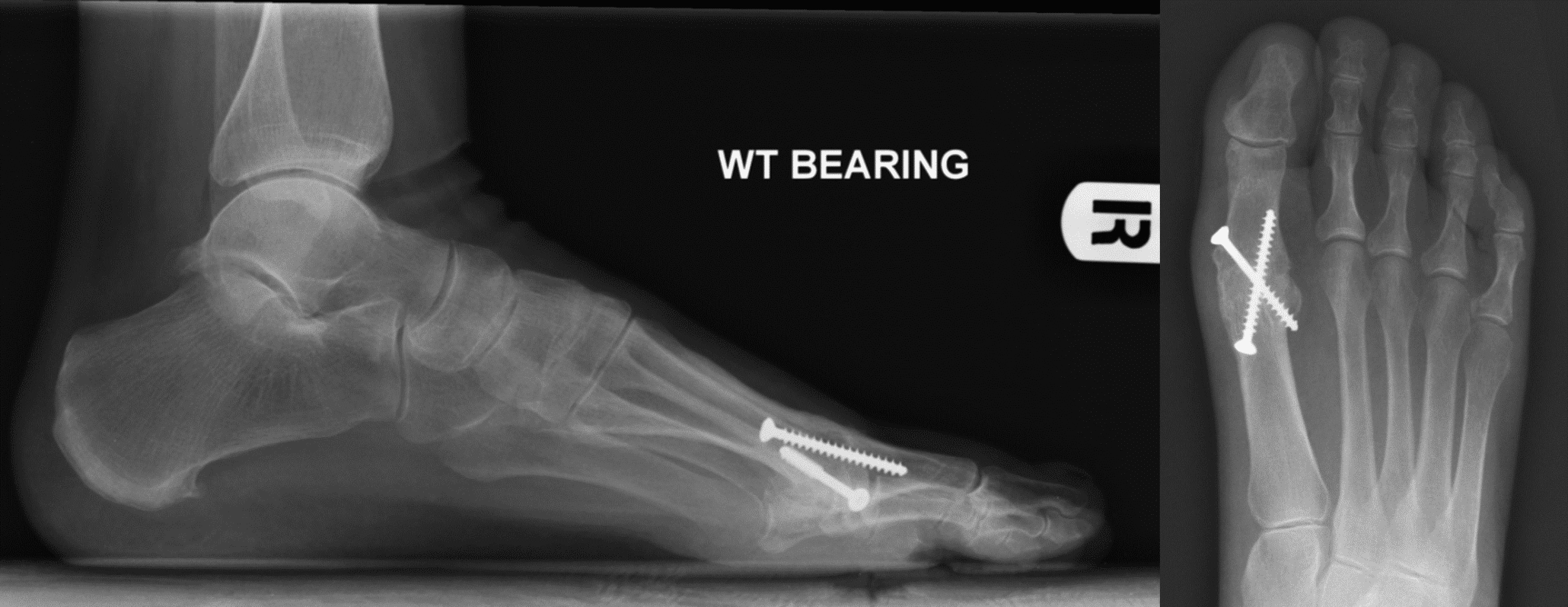
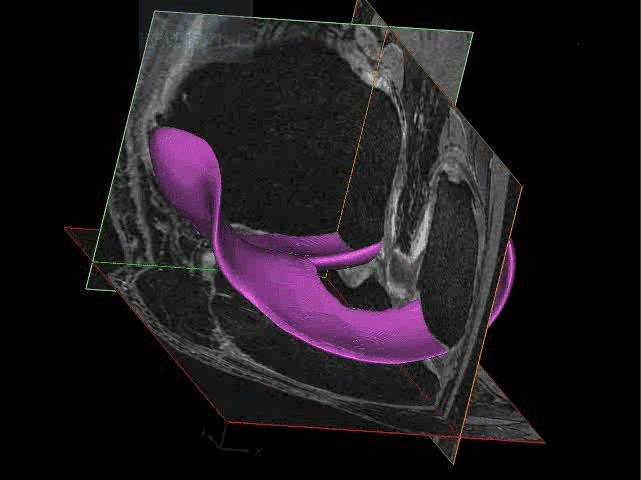
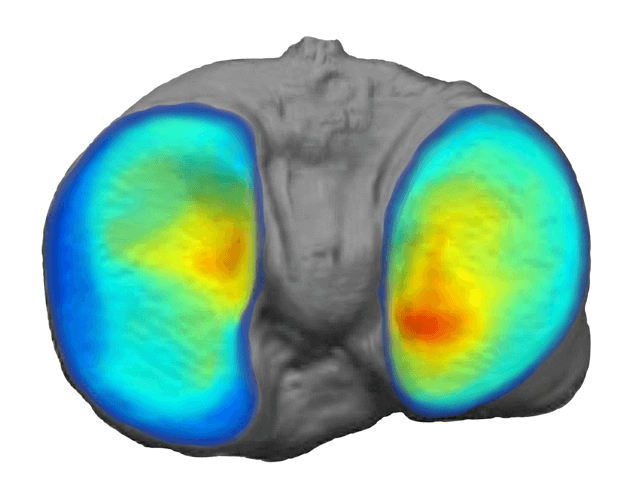
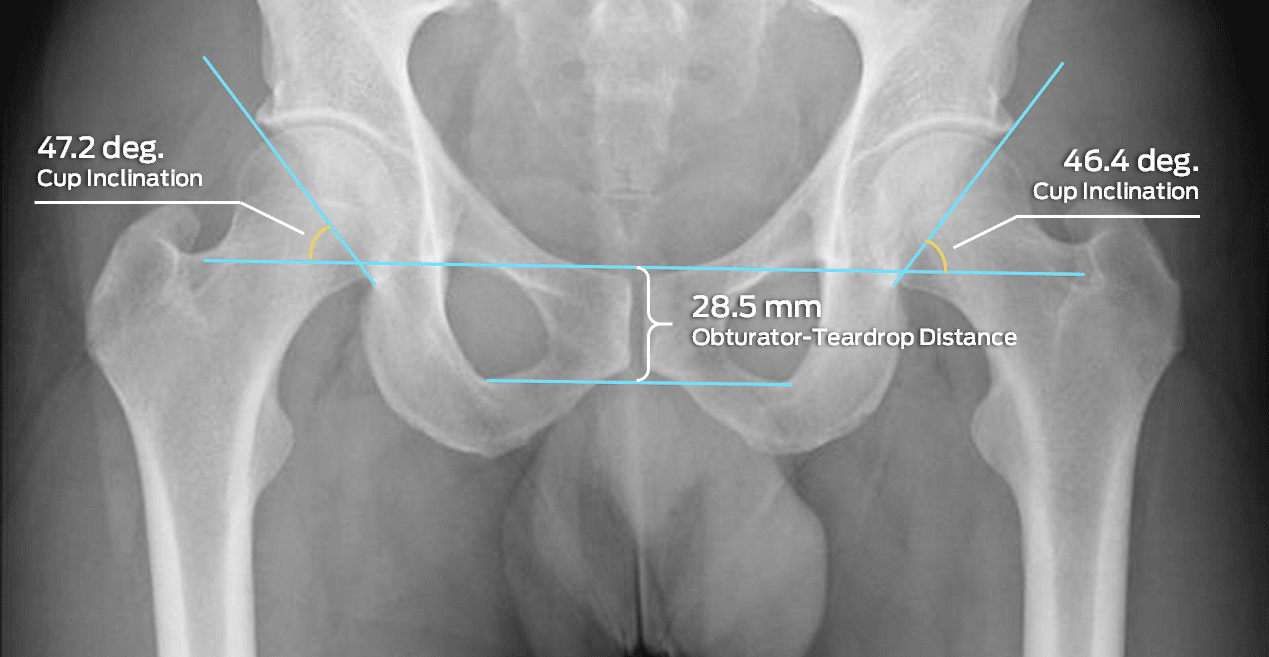
Some of Our Experts
Timothy Mosher, MD
Vice Chair of Radiology Research and Chief of MSK Radiology at Penn State University College of Medicine
Dr. Mosher has over 15 years’ clinical experience evaluating musculoskeletal imaging, and is a recognized expert in quantitative techniques for bone and soft tissue analysis, including cartilage relaxometry. He has published 50+ peer-reviewed papers and book chapters on musculoskeletal imaging, with an emphasis on MRI, and currently serves as the Associate Editor for Radiology, Osteoarthritis and Cartilage, Magnetic Resonance in Medicine, and is a frequent Instructional Course Lecturer for the American Academy of Orthopedic Surgeons (AAOS). Dr. Mosher is also a Member of the OARSI Working Group making recommendations to FDA on imaging techniques for OA trials.
William Morrison, MD
Professor of Radiology and Director of Musculoskeletal Radiology at Thomas Jefferson University Hospital
Dr. Morrison is an internationally recognized authority in musculoskeletal imaging with over 30 years’ experience. He has co-authored textbooks including Problem Solving in Musculoskeletal Radiology and Imaging of the Musculoskeletal System and published almost 30 book chapters and hundreds of peer-reviewed articles. Dr. Morrison has served on the editorial board of American Journal of Roentgeneology, Radiology, and Skeletal Radiology, on the Expert Panel on Musculoskeletal Radiology for the American College of Radiology (ACR), and the past president of the Society of Skeletal Radiology (SSR).
Relevant Scientific Resources
Key Publications
Documentation
MMI Oncology Brochure
Lorem ipsum dolor sit amet consectetur.
Spine CAMP™ Instructions for Use
Let MMI provide insights into your clinical study imaging.
Have questions? We’ll connect you immediately to one of our scientific managers and imaging experts. Your time is precious, and we want to make the most out of it.