MMI announces global imaging services collaboration with Micron
Learn MoreData Management & Quality Assurance
MMI EPIC™ Clinical Trial Platform
Imaging data at MMI are managed in a fully validated, 21 CFR Part 11 compliant, proprietary platform (MMI EPIC™) which was purpose-built for clinical trial workflow.
Imaging data at MMI are managed in a fully validated, 21 CFR Part 11 compliant, proprietary platform (MMI EPIC™) which was purpose-built for clinical trial workflow. From the instant that images are received at MMI, they are digitally tracked and monitored in our platform through every step of processing, query resolution, and analysis. All imaging and associated data are stored electronically, with audit trails and electronic signatures as required, and analysis results are digitally linked to the source images for full traceability. MMI EPIC™ has pre-programmed rules, queries, and redundancies built into the system to ensure that the appropriate images are always linked to the right subject and right imaging outcomes!


MMI EPIC™ Clinical Trial Platform
Imaging data at MMI are managed in a fully validated, 21 CFR Part 11 compliant, proprietary platform (MMI EPIC™) which was purpose-built for clinical trial workflow. From the instant that images are received at MMI, they are digitally tracked and monitored in our platform through every step of processing, query resolution, and analysis. All imaging and associated data
Imaging data at MMI are managed in a fully validated, 21 CFR Part 11 compliant, proprietary platform (MMI EPIC™) which was purpose-built for clinical trial workflow. From the instant that images are received at MMI, they are digitally tracked and monitored in our platform through every step of processing, query resolution, and analysis. All imaging and associated data are stored electronically, with audit trails and electronic signatures as required, and analysis results are digitally linked to the source images for full traceability. MMI EPIC™ has pre-programmed rules, queries, and redundancies built into the system to ensure that the appropriate images are always linked to the right subject and right imaging outcomes! Access to the system is strictly controlled via firewalls, physical security measures, and role-based logins. All data are managed at a secure colocation facility with redundant storage, fire suppression, and environmental controls to ensure that your data is safe and secure. Read more about our image storage and access services.
are stored electronically, with audit trails and electronic signatures as required, and analysis results are digitally linked to the source images for full traceability. MMI EPIC™ has pre-programmed rules, queries, and redundancies built into the system to ensure that the appropriate images are always linked to the right subject and right imaging outcomes! Access to the system is strictly controlled via firewalls, physical security measures, and role-based logins. All data are managed at a secure colocation facility with redundant storage, fire suppression, and environmental controls to ensure that your data is safe and secure. Read more about our image storage and access services.
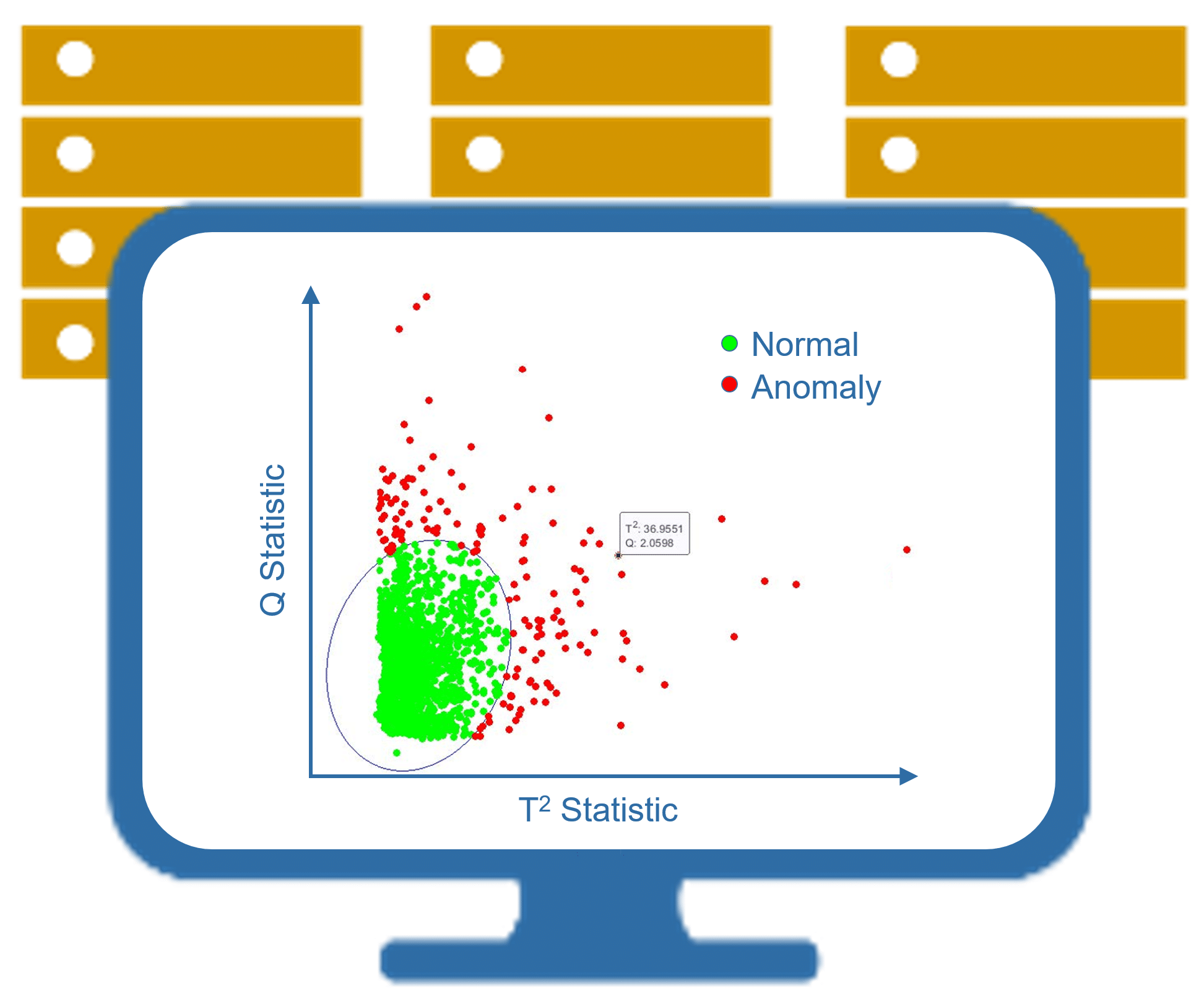
One of the many advantages of working with MMI is our comprehensive quality assurance program, which provides our clients with additional confidence that their imaging outcomes are accurate and reliable. Prior to the start of each study, our expert physician reviewers are carefully selected and go through a rigorous training program to prepare for the trial reads. During the study, our Technical Services team continuously scrutinize the data with custom-developed anomaly detection tools, project-specific, pre-programmed QC checks (e.g. for implausible data beyond physiological ranges), and additional inspections for data inconsistencies. We monitor reviewer agreement statistics and trends over time to make sure that the reported imaging outcomes are fully supported by the source images.
In addition to our custom-developed quality assurance programs for clinical trials, MMI is an ISO 9001:2015-certified company and committed to continuous improvement and delivering the highest standard of quality and reliability in our industry. Read more about MMI.
WHAT WE OFFER
Comprehensive Data Quality Assurance Program on a Reliable Clinical Trial Platform
Validated, 21 CFR Part 11 compliant database with multi-layer security and role-based access control
Electronic data capture, storage, and reporting with e-signatures and audit trails
Rigorous reviewer selection process and training programs
Study-specific inspections of image analysis data
Investigation of outliers, trends, and noteworthy findings
Systematic reliability testing and reader performance monitoring
ISO 9001:2015-certified core lab with CAPA program and emphasis on continuous improvement
How can we help?
Tired of paper CRFs and audit trails?
Want a partner who cares about data and scientific integrity as much as you do?
Looking for a secure and reliable way to manage your trial images?
Let MMI provide insights into your clinical study imaging.
Have questions? We’ll connect you immediately to one of our scientific managers and imaging experts. Your time is precious, and we want to make the most out of it.