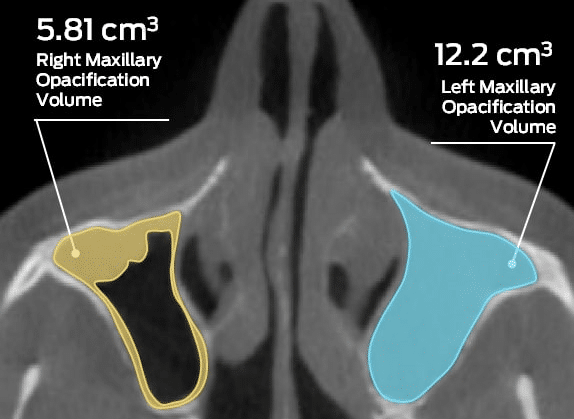
Today, it is becoming increasingly clear that having high-quality clinical data can provide a strategic advantage for medical device manufacturers. The extent and quality of clinical data has come to define a device’s reception in the marketplace, and industry stakeholders and patients alike are demanding greater transparency and increased evidence of efficacy. The quality of a device’s clinical data serves to set it apart from competing options, dramatically increasing the likelihood of widespread adoption.
However, some device makers have been slow to adapt to this changing landscape. In many cases, producing this larger body of evidence requires outsourcing to a contract research organization (CRO), as manufacturers themselves lack the tools, bandwidth and experience necessary to successfully run a clinical trial themselves. This level of trust often does not come easily, and many manufacturers are intimidated by the prospect of clinical outsourcing.
Yet with the proper consideration and a clear understanding of what you value in a CRO, it is possible to develop an outsourcing strategy that can get your product to market more efficiently and with greater evidence to support it. Especially if your company lacks experience in medical device trials, partnering with an experienced CRO can help ensure the approval pathway is navigated properly. Even with certain 510(k) applications, where clinical evidence isn’t mandated, having thorough supporting data can help differentiate your product.
The key is to select a CRO with well-documented expertise in medical device clinical trials. Pharmaceutical trials and medical device trials are significantly different, and each require specific expertise. It is best to be open and fully transparent with your CRO, and to share your expectations and needs clearly and as early in the process as possible. The importance of transparency, honesty and trust can not be overstated in the relationship, as these will affect nearly every aspect of the trial.
As such, your chosen CRO should be upfront about their capabilities, expertise and resources. This results in a more accurate project scope, and reduces the likelihood of confusion over responsibilities and services provided. By conducting this due diligence, project planning is more successful, and both parties can share aligned priorities.
Medical Metrics has a extensive history of product approvals dating back to our founding in 2000. Our clinical and scientific experts are recognized leaders in orthopedics, spine, neurology, cardiology and vascular medicine, and are eager to consult with your on the optimal imaging strategies for your upcoming trial.
Latest Industry News
[Press Release] Driving Excellence in Cardiovascular Trials: Medical Metrics, Inc and Healthcare Inroads, LLC Deepen Collaboration
Medical Metrics, Inc. Announces Involvement in ReOpen CRS Clinical Trials