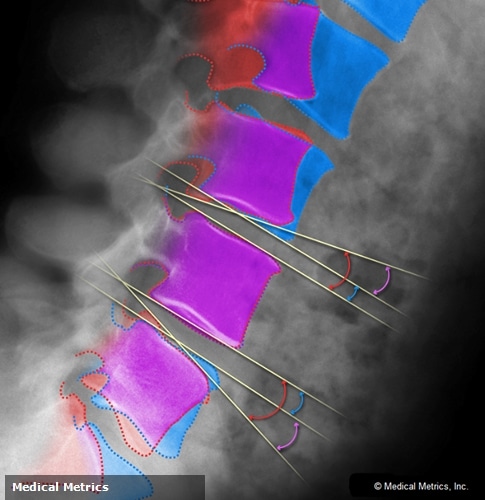
Medical Metrics, Inc. (MMI) announces the publication of the 150th scientific report featuring its Quantitative Motion Analysis (QMA®) technology. QMA® is MMI’s proprietary radiographic measurement and image enhancement technology that has been extensively validated and used in numerous clinical and research studies of treatments for spinal disorders. Further, QMA® has also been used in many US-Food and Drug Administration (FDA)-regulated clinical trials studying such treatments and is 510(k)-cleared by the FDA.
The list of scientific publications featuring QMA® spans leading peer-reviewed journals such as Spine, The Spine Journal, SAS Journal, Journal of Spinal Disorders and Techniques, Journal of Neurosurgery: Spine, Journal of Biomechanics, etc. In addition, several posters and podium presentations involving image analysis with QMA® have been made at prestigious scientific fora such as the annual meetings of the North American Spine Society (NASS), Spine Arthroplasty Society (SAS) / International Society for the Advancement of Spine Surgery (ISASS), EuroSpine, Cervical Spine Research Society (CSRS), etc. Some key publications include:
- A study showing that QMA® based approach substantially improved classification of instability and measurements of lumbar intervertebral motion: Pearson AM, Spratt KF, Genuario J, McGough W, Kosman K, Lurie J, Sengupta DK. Precision of lumbar intervertebral measurements: Does a computer-assisted technique improve reliability? Spine 2011.
- A study showing that QMA®-based motion analysis is less subjective and more predictive than CT-based evaluation of pseudoarthroses: Ghiselli G, Wharton N, Hipp JA, Wong DA, Jatana S. Prospective Analysis of Imaging Prediction of Pseudarthrosis After Anterior Cervical Discectomy and Fusion. Spine 2011; 36(6):463-468.
- A Book Chapter authored by MMI staff members, published in Motion Preservation Surgery of the Spine: Advanced Techniques and Controversies. Edited by Yue J, Bertagnoli R, McAfee P, and An H. Elsevier, New York, 2008: Hipp JA and Wharton ND. Quantitative Motion Analysis (QMA®) of Motion Preserving and Fusion Technologies for the Spine.
- An independent validation study of the accuracy of QMA® performed by researchers at the Mayo Clinic, Rochester, MN: Zhao KD, Yang C, Zhao C, Stans AA, An K-N. Assessment of noninvasive intervertebral motion measurements in the lumbar spine. Journal of Biomechanics 2005; 38:1943-1946.
QMA® continues to gain widespread acceptance in pre-clinical, clinical and research applications studying intervertebral motion as evidenced by the growing number of scientific publications. QMA®-derived data have been accepted by the FDA as a part of clinical trials involving motion-preserving devices and fusion treatments, including several “firsts to market”. With measurement accuracy validated to within 0.5 degrees and 0.5 mm, this technology is widely regarded as the “gold standard” for quantitative intervertebral motion analysis.
About Medical Metrics
Medical Metrics, Inc. (MMI) is an experienced, ISO 9001-certified, independent imaging core lab for multi-center clinical trials. Since 2000, MMI has partnered with clinical trial sponsors to conduct comprehensive imaging evaluations of treatments for musculoskeletal, cardiovascular, and neurological disorders. Trials range from pre-clinical and early-phase clinical studies to pivotal trials and post-approval surveillance. With an ISO-certified quality system, experienced project management team, proprietary data management platform, and luminary group of radiology and cardiology thought-leaders, MMI is uniquely positioned to deliver high-quality imaging core lab services for complex clinical trials.
Latest MMI News & Events
[Press Release] Driving Excellence in Cardiovascular Trials: Medical Metrics, Inc and Healthcare Inroads, LLC Deepen Collaboration
MMI CSO, Dr. John Hipp Selected as Guest Editor of Bioengineering Special Issue! - Submissions Due May 30 (Updated)