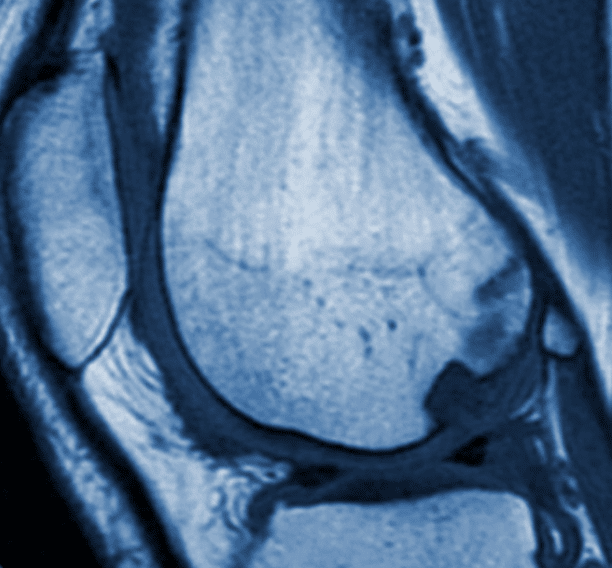
Medical Metrics, Inc. (MMI), an experienced provider of medical imaging core lab services for global clinical trials, has been selected by Exactech, Inc. to conduct an independent evaluation of medical images from the BiPhasic Cartilage Repair Implant (BiCRI) IDE Clinical Trial approved by the Taiwan FDA. This prospective, randomized study will be conducted in Taiwan to evaluate a novel cartilage repair treatment. The biphasic implant is a two-phase scaffold that is intended to promote integration with subchondral bone and regeneration of hyaline-like cartilage. During the study, MMI will provide comprehensive analysis and management of MR images.
MMI developed the imaging sequences and assessment panels necessary to evaluate treatment outcomes, including extent of lesion repair, bone-implant integration, and overall device integrity. Matt Christensen, Exactech’s Director of Clinical Affairs, said “We chose Medical Metrics for their extensive expertise in musculoskeletal imaging and cartilage repair. MMI is a valuable resource for clinical input on study design, and their commitment to customer services is impressive.”
Exactech’s BiCRI IDE trial supports the company’s corporate objective to enter the cartilage repair market and commercialize the new technology in multiple global markets. MMI has supported osteoarthritis (OA) trials of treatments for cartilage repair and replacement since 2000. Nick Wharton, MMI’s SVP of Technical Services, commented “Sponsors benefit from our broad experience in OA clinical trials and unique clinical and scientific expertise. We are proud to have been selected by Exactech as the imaging core lab for this important pivotal trial.”
About Medical Metrics
Medical Metrics, Inc. (MMI) is an experienced, ISO 9001-certified, independent imaging core lab for multi-center clinical trials. For more than 15 years, MMI has partnered with clinical trial sponsors to conduct comprehensive imaging evaluations of treatments for musculoskeletal, cardiovascular, and neurological disorders. Trials range from pre-clinical and early-phase clinical studies to pivotal trials and post-approval surveillance. With an ISO-certified quality system, experienced project management team, proprietary data management platform, and luminary group of radiology and cardiology thought-leaders, MMI is uniquely positioned to deliver high-quality imaging core lab services for complex clinical trials.
About Exactech
Based in Gainesville, Fla., Exactech develops and markets orthopaedic implant devices, related surgical instruments and biologic materials and services to hospitals and physicians. The company manufactures many of its orthopaedic devices at its Gainesville facility. Exactech’s orthopaedic products are used in the restoration of bones and joints that have deteriorated as a result of injury or diseases such as arthritis. Exactech markets its products in the United States, in addition to more than 30 markets in Europe, Latin America, Asia and the Pacific. Additional information about Exactech, Inc. can be found at http://www.exac.com.
Latest MMI News & Events
[Press Release] Driving Excellence in Cardiovascular Trials: Medical Metrics, Inc and Healthcare Inroads, LLC Deepen Collaboration
MMI CSO, Dr. John Hipp Selected as Guest Editor of Bioengineering Special Issue! - Submissions Due Nov 30