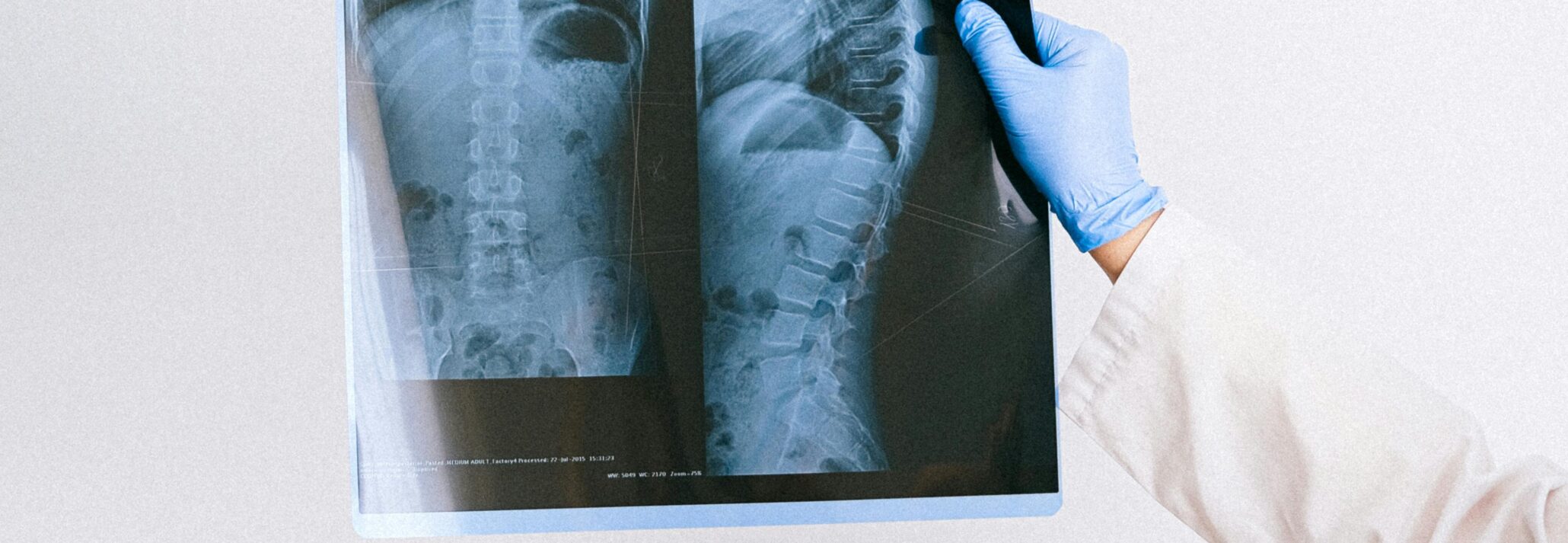
Radiographic spine fusion assessment is complicated by a lack of standardized criteria, highlighting the need for imaging expertise during clinical trials.
Key Highlights:
- The primary outcome measure for lumbar interbody fusion devices is typically radiographic fusion.
- A recent review article identified over 250 unique fusion criteria combinations and 18 fusion definitions, presenting challenges to sponsors comparing fusion outcomes between studies and assessing novel treatments.
- MMI’s extensive experience in spine clinical trials and Quantitative Motion Analysis (QMA®) technology enhances the reliability, accuracy and value of radiographic fusion assessment results.
Radiographic fusion assessments are complex and vary across studies:
Lumbar interbody fusion technologies have advanced significantly in recent decades, with the development of novel devices and materials to improve treatment outcomes continuing today. The primary outcome measure for lumbar fusion clinical trials is typically fusion assessed on radiographs, as the FDA continues to rely heavily on imaging-based criteria in spinal system guidance documents for IDE applications.
Given this reliance on imaging, it may come as a surprise that there are no widely accepted criteria for assessing radiographic lumbar fusion. A recent review article in JBJS Reviews reports on the wide range of interbody fusion assessment strategies and summarizes available literature on the reliability and accuracy of these assessments.
Duits AAA, van Urk PR, Lehr AM, et al. Radiologic Assessment of Interbody Fusion: A Systematic Review on the Use, Reliability, and Accuracy of Current Fusion Criteria. JBJS Rev. 2024;12(1):e23.00065. Published 2024 Jan 9. doi:10.2106/JBJS.RVW.23.00065.
A total of 442 publications dating back to 1968 reported fusion method in detail. Several approaches (including ALIF, PLIF, and TLIF) and implant types (including radiolucent and radiopaque cages and grafts) were included, and the majority of studies supplemented interbody fusion with posterior fixation. These publications identified 18 definitions of radiographic interbody fusion and 256 unique combinations of assessment methods. This excess of fusion criteria presents a major challenge for sponsors, limiting their ability to compare new spine fusion treatments to previously published outcomes.
Considerations during spine clinical trials:
With over 20 years of experience in hundreds of spine clinical trials, MMI has worked with nearly every fusion assessment strategy and definition mentioned in this this extensive article review. MMI provides specific image acquisition and analysis recommendations for each clinical trial based on certain key considerations below.
The first factor to consider is which imaging modalities to acquire and utilize for fusion assessment. According to the review article, less than 20% of lumbar fusion studies used computed tomography (CT) scans before 2013. Since then, CT has become the most popular modality (36%), though conventional radiographs (32%) and dynamic (i.e. flexion/extension) radiographs (32%) are still commonly used. The type of construct, materials, and presence of additional hardware are also important to consider. When available, MMI’s imaging analysts and reviewers leverage all three modalities when assessing fusion.
MMI has lead the way for analysis of dynamic spine radiographs with its patented Quantitative Motion Analysis (QMA®) technology, which has been referenced in over 200 publications and is considered the gold standard for quantitative spine measurements. In the aforementioned review article, angular motion of <5° was the most common threshold for assessing fusion. QMA’s accuracy of <0.5° and 0.5 mm enables measurements well below this threshold.
Selecting qualitative fusion criteria from the 57 descriptive fusion criteria identified in the review article presents another challenge. Continuity of bony bridging in the disc space and radiolucency around the cage were the most common qualitative measures as the only criteria chosen in over 75% of the studies. QMA® enables enhanced assessment of these qualitative measures through the semi-automated registration or “stabilization” of images from each patient. QMA® stabilized dynamic radiographs allows radiologists or other imaging reviewers to toggle scaled and overlaid flexion/extension images, while QMA stabilized conventional radiographs allow them to toggle images across early and late-stage imaging visits. With QMA®, reviewers can more confidently assess if bridging bone is solid and whether radiolucencies are progressing over time.
The review article also suggests CT-based continuity of bony bridging in the disc space lends itself to additional insight during fusion assessment, as 3D visualization of irregularly shaped, immature fusion masses seems to overestimate fusion less than 2D imaging. MMI’s reviewers are highly experienced with bony bridging assessments and routinely perform them using both CTs and conventional radiographs. MMI inspects observer agreement between reviewers for assessments including continuity of bony bridging and radiolucency, and finds strong inter-observer reliability consistent the review article.
How MMI can help:
The lack of standardized criteria and complexity in radiographic spine fusion assessment presents a major challenge for sponsors. As the leading imaging core lab for spine clinical trials, MMI provides guidance and confidence within this complex landscape. In addition to its proprietary QMA® technology, MMI offers endpoint consulting, imaging protocol development, experienced reviewers, and scientific consulting and interpretation, enhancing the reliability, accuracy and value of your spine study results.
Medical Metrics, Inc. is an ISO 9001:2015-certified provider of independent imaging core lab services and a leading core lab for spine and orthopedics clinical trials and related consulting. We assist sponsors with designing, implementing, and executing the imaging strategy of their global trials through our responsive trial management team, robust operating infrastructure, and world-class imaging expertise. Learn more about our Spine expertise and our Services.